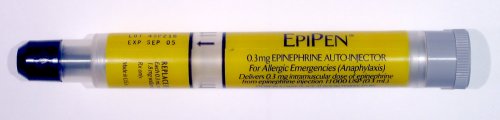
 Federal regulators at the Food and Drug Administration (FDA) have announced the approval of the first generic epinephrine auto-injector. Manufactured by Israel-based Teva Pharmaceutical, this concludes a lengthy approval process undertaken by drug manufacturers before their product can be marketed to consumers. In a press release, FDA Commissioner Scott Gottlieb, M.D. said, “Today’s approval of the first generic version of the most-widely prescribed epinephrine auto-injector in the U.S. is part of our longstanding commitment to advance access to lower cost, safe and effective generic alternatives once patents and other exclusivities no longer prevent approval.” A 2014 study in the Journal of Allergy and Clinical Immunology found that upwards of 1 in 20 Americans may be susceptible to anaphylaxis, an acute, life-threatening allergic reaction to specific foreign substances. When asked about future plans for responding to anaphylactic episodes, 1 in 3 patients in the study planned on self-administering epinephrine using an auto-injection device. This is no surprise, as many medical organizations consider intramuscular injection of epinephrine as the first line of defense against anaphylactic shock. By incorporating precise doses of epinephrine into a compact and user-friendly device, patients with known susceptibility to anaphylaxis can quickly treat themselves after exposure to an allergen, which could include specific medications, foods, or insect stings. Although this technology has been on the market for decades, epinephrine auto-injectors have recently received scrutiny due to their scarcity and high cost. Mylan, a prominent pharmaceutical company, became engulfed in controversy in 2016 after the price of its proprietary auto-injector, the notorious “EpiPen”, increased by over 400%. Teva Pharmaceuticals was the largest manufacturer of generic pharmaceuticals in 2010 and has a long history of developing generic alternatives to brand-name drugs that are on the market. There is a hope among those at risk for anaphylaxis that Teva’s cheaper, generic alternative will put an end to the controversy and make this crucial device more affordable and accessible.
Federal regulators at the Food and Drug Administration (FDA) have announced the approval of the first generic epinephrine auto-injector. Manufactured by Israel-based Teva Pharmaceutical, this concludes a lengthy approval process undertaken by drug manufacturers before their product can be marketed to consumers. In a press release, FDA Commissioner Scott Gottlieb, M.D. said, “Today’s approval of the first generic version of the most-widely prescribed epinephrine auto-injector in the U.S. is part of our longstanding commitment to advance access to lower cost, safe and effective generic alternatives once patents and other exclusivities no longer prevent approval.” A 2014 study in the Journal of Allergy and Clinical Immunology found that upwards of 1 in 20 Americans may be susceptible to anaphylaxis, an acute, life-threatening allergic reaction to specific foreign substances. When asked about future plans for responding to anaphylactic episodes, 1 in 3 patients in the study planned on self-administering epinephrine using an auto-injection device. This is no surprise, as many medical organizations consider intramuscular injection of epinephrine as the first line of defense against anaphylactic shock. By incorporating precise doses of epinephrine into a compact and user-friendly device, patients with known susceptibility to anaphylaxis can quickly treat themselves after exposure to an allergen, which could include specific medications, foods, or insect stings. Although this technology has been on the market for decades, epinephrine auto-injectors have recently received scrutiny due to their scarcity and high cost. Mylan, a prominent pharmaceutical company, became engulfed in controversy in 2016 after the price of its proprietary auto-injector, the notorious “EpiPen”, increased by over 400%. Teva Pharmaceuticals was the largest manufacturer of generic pharmaceuticals in 2010 and has a long history of developing generic alternatives to brand-name drugs that are on the market. There is a hope among those at risk for anaphylaxis that Teva’s cheaper, generic alternative will put an end to the controversy and make this crucial device more affordable and accessible.
Home Epinephrine Auto-Injector Cheaper Treatment on the Way: FDA Approves First Generic Epinephrine Auto-Injector


